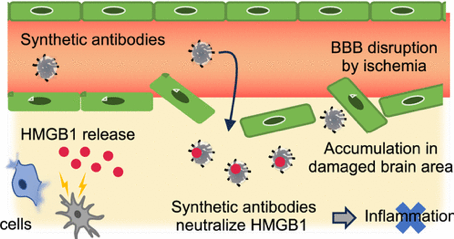
A synthetic antibody (SA) produced by Shea and coworkers has been found to reduce brain damage resulting from from cerebral ischemic reperfusion injury. The synthetic antibody targets high-mobility group box 1 (HMGB1), a multifunctional protein that upon injury or infection, is passively released from necrotic and activated dendritic cells and macrophages, where it functions as a cytokine, acting as a ligand for RAGE, a major receptor of innate immunity stimulating inflammation responses including the pathogenesis of cerebral ischemia/reperfusion (I/R) injury. In vitro studies established that the anti-HMGB1-SA inhibits HMGB1 interaction with the RAGE receptor. Using temporary middle cerebral artery occlusion (t-MCAO) model rats, anti-HMGB1-SA was found to accumulate in the ischemic brain by crossing the blood−brain barrier. Significantly, administration of anti-HMGB1-SA to t- MCAO rats dramatically reduced brain damage caused by cerebral ischemia/reperfusion. The knowledge gained from these experiments can facilitate the discovery, design, and development of a new category of drug.
Design of an Anti-HMGB1 Synthetic Antibody for In Vivo Ischemic/Reperfusion Injury Therapy
